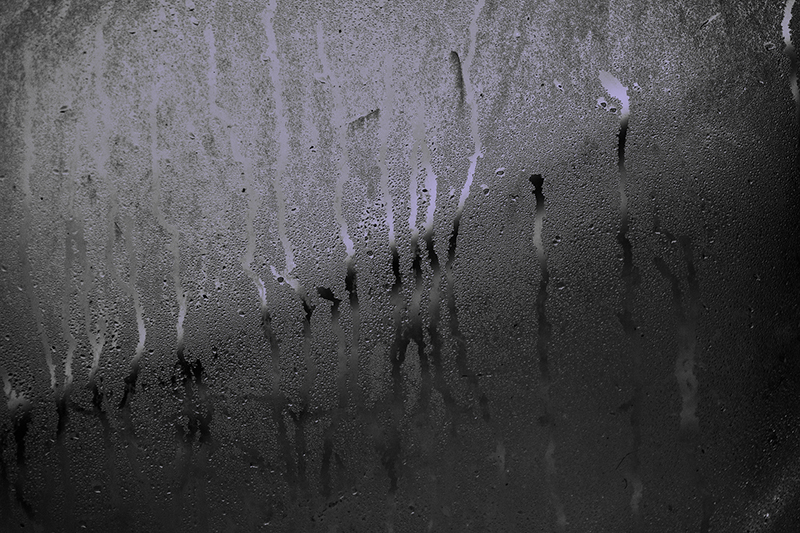
Outside condensation on newer windows
Newer double and triple glazing offers many benefits:
- Retaining far more heat within the home
- reducing the time your heating needs to be on
- Keeping the rooms at a more stable internal temperature
- Reducing condensation on the glass (because the internal pane is much warmer, so the moisture in the air doesn’t condense on it, helping keep the windows mist-free)
Condensation forming between the panes of glass is common in slightly older double glazing – because the airtight seal on the unit has broken down, allowing air (and moisture) to enter the space between the two pains.
As the quality of glazing improves condensation is increasingly appearing on the very outside of the window.
Why is there sometimes condensation on the outside of new double and triple glazing?
Condensation forming on the outside of new double/triple glazing is a natural occurence, because the window is working so well at preventing heat loss from within your home.
Note: Condensation is the process by which a gas turns into a liquid. If the temperature of an object falls below what is known as the dew point temperature, then water vapour from the air will condense on the surface. The dew point varies according to the amount of water in the air (compare a shower room to a sitting room for example) and the temperature of the air – the warmer the air, the more water vapour it can hold – but it can only hold so much, if this saturated air encounters a surface that is below the dew point temperature then it naturally condenses.
Water condenses on the outside surface of good quality glazing systems if the temperature of the glass drops below the external dew point temperature. New double or triple glazing units tend to have inner panes made up of low emissivity glass and this prevents the movement of heat across the glazing unit, so the outer pane never gets warm.
Condensation on the outside of your new windows is actually an indication that your new windows are performing very well, although we appreciate it may not seem ideal!